
With more than 5,000 installed machines, Fette Compacting is the international market leader in the area of tablet presses in the pharmaceutical industry. Fette Compacting is based in Schwarzenbek, near Hamburg, and is a member of Excellence United — an alliance in the construction of special machines for the pharmaceutical industry. The area of containment research in pharma is of critical importance for the safety of machine operators, to avoid them being contaminated with medicinal residues which are airborne. Fette Compacting has employed CFD to advance its testing methodology.
Containment research has been trying to better understand the dust dispersion of pharmaceutical powders containing active pharmaceutical ingredients (APIs). However, results from exposure measurements can rarely be transferred to other facilities. A novel dual-chamber model developed by the University of Hamburg together with Fette Compacting hopes to change this. The pharmaceutical industry is increasingly focusing on machine operator protection and quality assurance. Fette Compacting has therefore set itself the task of stepping up systematic research into more efficient containment systems, specifically by developing the dual-chamber model. The dual-chamber model takes into account how air flows within the machine chamber influence dust dispersion. The new design is also of interest to the nutritional and chemicals industries that widely employ Fette Compacting machines in product manufacturing.
Containment measurements are very time-consuming and therefore costly. Moreover, they have to be carried out anew every time a different active ingredient is to be processed on the machine. If we succeed in standardizing relevant measurement factors, and thus making the results transferable, this holds enormous potential for cost savings.

Steffen Wirth
Researcher at The University of Hamburg, Germany
Steffen Wirth is a research associate at the Institute of Pharmacy at the University of Hamburg. He studied pharmacy at the Christian-Albrechts-Universität zu Kiel from 2013 to 2017. After completing his studies, Wirth completed pharmacy internships at the University of Copenhagen and in Hamburg. Since November 2018, he has been working at the University of Hamburg. His research focuses on his dissertation, which deals with the “Investigation of the powder characteristics of tabletting materials with special consideration of dust development in containment technology.”
The dust behavior of a pharmaceutical powder depends on many factors. The shape, size, and distribution of the particles, the humidity of the powder and the ambient air, as well as electrostatic charges within the machine are only some of the relevant influencing variables. Another important role is played by the mass transport through which the dust is distributed in the surrounding space. Measurement results from different manufacturing plants can not be transferred to other plants in most cases, or only to a very limited extent. The reason for this is simple: as a rule, for safety reasons, the tests are carried out with substitute substances whose physical properties are different from the actual pharmaceutical products. Therefore, for safety reasons, it would be careless to assume that results from one installation might be valid for any other.
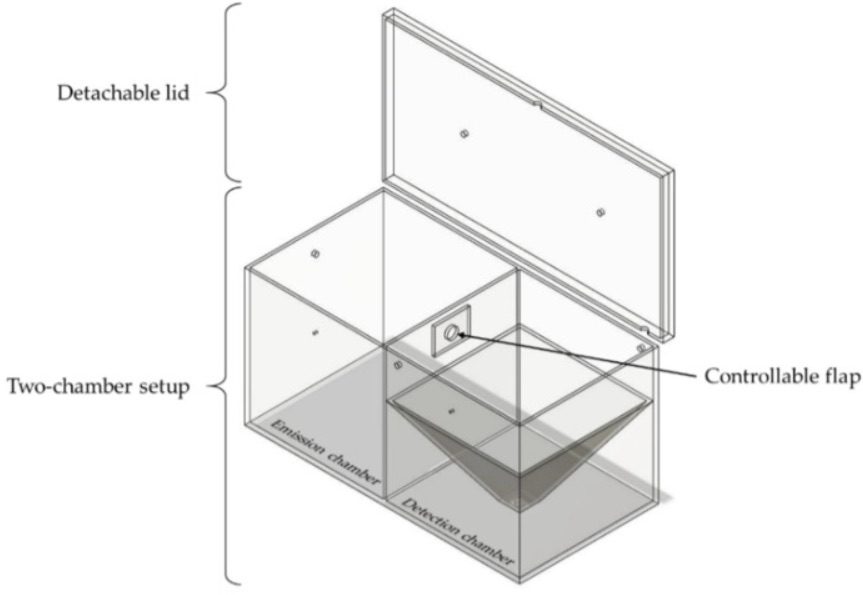
This is where the dual-chamber model comes into play. The apparatus is divided into two areas separated by a closed flap. In the emission chamber, a reproducible negative pressure overpressure is used to first atomize a small amount of powder. After atomization, the chambers are connected by opening the flap for one minute, when a certain amount of the powder dust passes into the adjacent detection chamber. There, the particle concentration of the ambient air is determined by a measuring device installed at the bottom of the chamber.
Jonas Brügmann is a Mechanical Engineer at Fette Compacting whose MSc thesis was on using CFD to model Fette Compacting producing tablet presses for the pharmaceutical industry. His goal was to reproduce the air and particle dust flow behavior inside and around the machines and their impact on human operators to ensure that APIs won’t be inadvertently breathed in. Most airborne dust particles from the machines contain active medicinal ingredients and hence quantifying exposure is of critical importance to the occupational health of the operators.
We found using SimScale to be very easy and the UI was much more intuitive compared to other desktop simulation software. A big benefit to adopting SimScale was its cloud deployment and simple access from a web browser. Due to the cloud-based approach, my PC is not busy processing the simulations and is free to be used in the meantime.

Jonas Brügmann
Mechanical Engineer at Fette Compacting
Fette Compacting used computational fluid dynamics (CFD) to analyze the flow and air mixing behavior of the contaminant dust in the chambers, including the mechanism of transport between the two chambers and deposition onto a machine operator. The results were compared to in-situ physical data from the chamber and the IOM filter data that represents the risk to machine operators. The key outputs from CFD were air velocities, mixing efficacy, and residence time of the dust particles in the chamber. CFD was used to determine the evacuation time or residence time of the dust in the chambers. A time period of 9 min was sufficient for a reproducible evacuation and reliable detection of most airborne particles within the detection chamber. Comparisons to actual measured data using smoke tests showed a high degree of validation of the CFD setup.
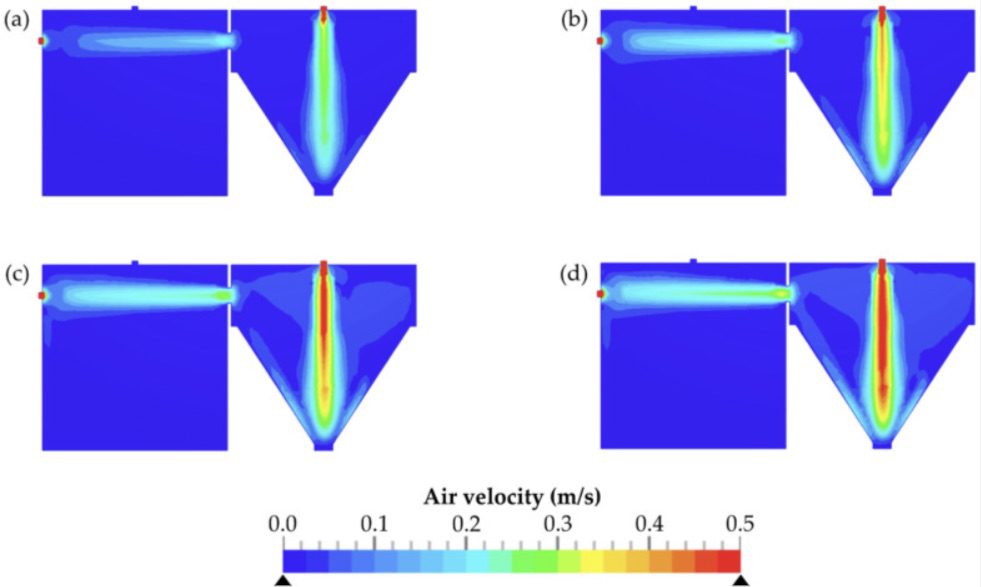
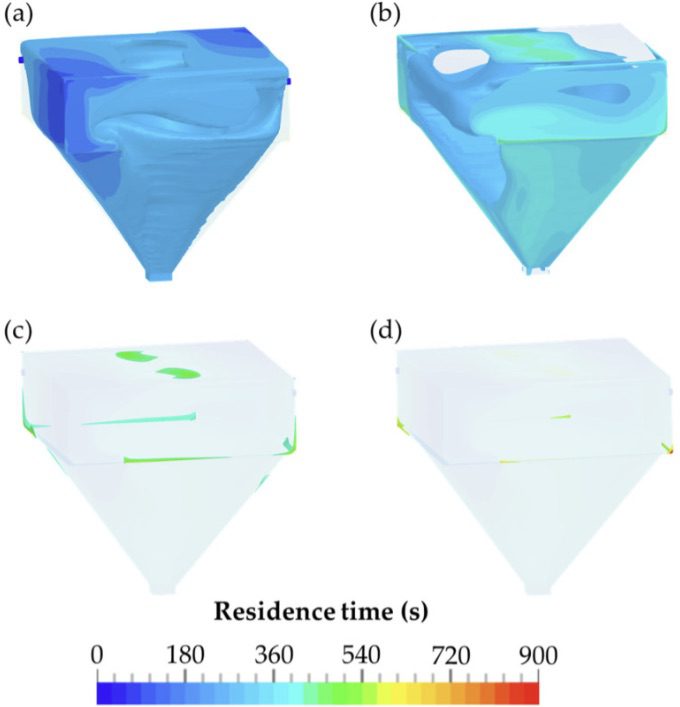
Before investing in using SimScale, the standard methodology was to perform physical tests on purposely built test rigs that are costly and time-consuming. Each new deployment with a new pharmaceutical product needed testing according to international SMEPAC standards. Sampling using patch filters on operators and inside the machines is a common practice utilizing an Institute of Occupational Medicine (IOM) sampler that has a collection method that closely simulates the manner in which airborne workplace particles are inhaled through the nose and mouth.
The dual-chamber system is currently at the research stage and undergoing further development using SimScale’s CFD and structural analysis types. The eventual goal is to standardize CFD simulations as part of the testing process and obviate a large amount of the costly physical testing.
One of the standout features of SimScale is its collaboration tools. As a mechanical engineer, I was able to share the project and discuss the results with my colleagues who are pharmacists and machine operators. They could easily access the project and quickly understand the results. This helped us to communicate more effectively as an interdisciplinary team with SimScale as the enabler. We are now looking forward to making use of the other analysis types in SimScale, including FEA.

Jonas Brügmann
Mechanical Engineer at Fette Compacting

Sign up for SimScale
and start simulating now